Ernest Rutherford

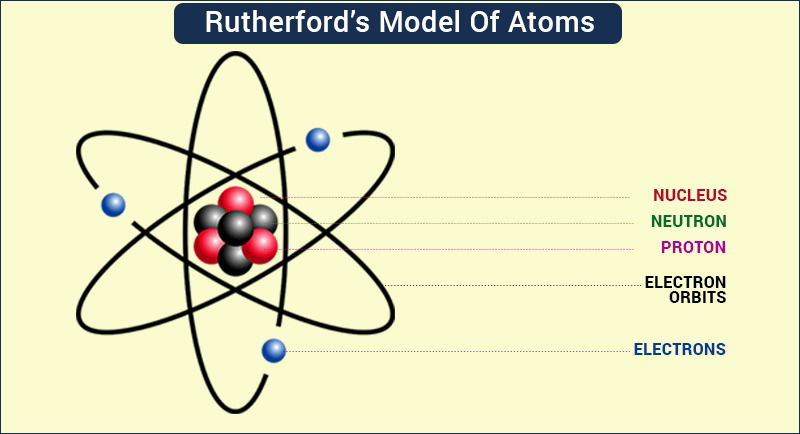
Scientists already had a clear picture that all objects were made of atoms. Rutherford's theory built on that knowledge, claiming that the atom is not one solid substance, but that it's made of different parts--a nucleus with positively charged protons and neutral neutrons surrounded by negatively charged electrons. To see if atoms were one big particle or if they were made of multiple smaller particles, Rutherford experimented with pure gold foil.
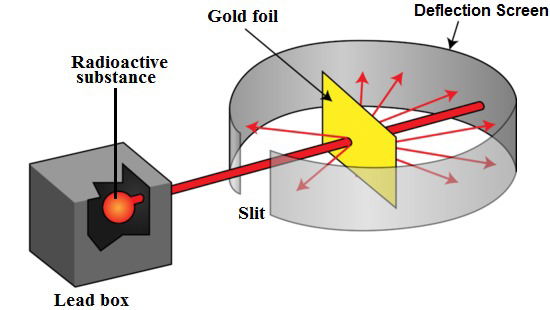
He passed what he called alpha particles through the foil (an alpha particle is the same as the nucleus of helium atom). If atoms were one solid particle, they would be packed together like a bag of marbles. The alpha particles, which are smaller, would pass between the atoms and come out the other side. However, if there was an object in the center of the atom, some of the alpha particles would be bounced back. This last scenario is what occurred. The alpha particles were bouncing back because they were hitting the nucleus of the gold atom, proving that an atom is made up of different parts!
